The driving goal of our laboratory is to define immune mechanisms in chronic inflammatory disorders and cancer, while working towards identifying novel biomarkers of disease and therapeutic interventions. We utilize gene targeting in mouse models of disease, clinical patient samples, and relevant cell culture systems to study the cellular and molecular basis of immune-mediated disorders.
We are particularly interested in understanding the role of microRNAs (miRNAs) in chronic inflammatory disorders and cancer. The discovery of miRNAs has led to a paradigm shift in our understanding of gene regulation mechanisms. MiRNAs are a class of small noncoding RNAs that post-transcriptionally control the levels of protein-coding genes in all animals. The human genome encodes over 1000 different miRNAs which regulate more than 70% of protein-coding genes. Because of their ubiquity, miRNAs are involved in virtually all biological processes and their dysregulation has been associated with a wide range of diseases.
As a part of the Ann Romney Center for Neurologic Diseases, a primary focus of our lab involves miRNAs in multiple sclerosis (MS), an autoimmune disease of the central nervous system (CNS). We have identified pathogenic roles for the microRNAs, miR-155 and miR-21, in inflammatory Th1 and Th17 cell differentiation and function in MS and its animal model, experimental autoimmune encephalomyelitis (EAE). Importantly, we have shown silencing miR-155 and miR-21 with therapeutic inhibitors can reduce paralysis in animals with EAE (The Journal of Immunology, 2010; The Journal of Clinical Investigation, 2015).
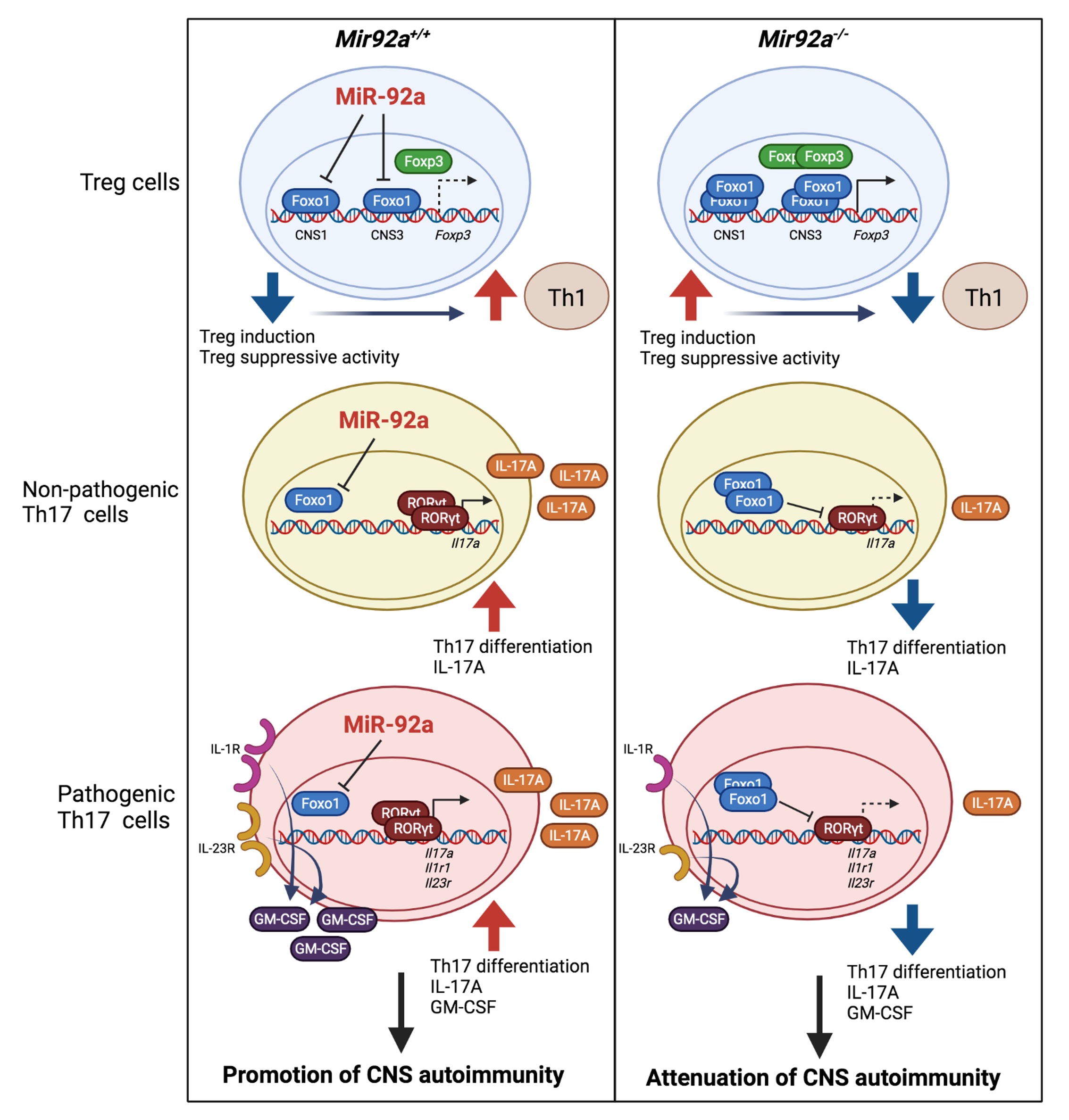
Although we and others have associated miRNA function in EAE with miRNA dysregulation observed in MS patients, finding specific miRNA pathways that directly connect clinical disease activity in MS patients with immune mechanisms has remained a challenge. Recently, we discovered a functional mechanism for a clinically relevant miRNA, miR-92a, whose expression is elevated in MS and is strongly associated with worsening disease activity and harmful immune mechanisms in MS. Using gene-deficient animals and MS patient samples, we identified how miRNA-92a promotes MS pathogenesis by altering the balance of inflammatory Th1/Th17 and regulatory T cells (The Journal of Clinical Investigation, 2022).Currently, we are exploring the role of other clinically relevant miRNAs in MS pathogenesis and testing miRNA-based disease-modifying therapies in MS animal models and MS patient samples. These studies are informed by miRNA profiles in MS patients which correlate with neurologic impairment and brain atrophy. Because women are 3-4 times more likely to develop MS than men, we are also interested in studying the role of miRNAs in the MS gender bias. We have identified sexually dimorphic MS miRNA profiles in circulation and are currently testing our hypothesis that the female preponderance in MS is influenced by differentially expressed miRNAs affecting key immune mechanisms.
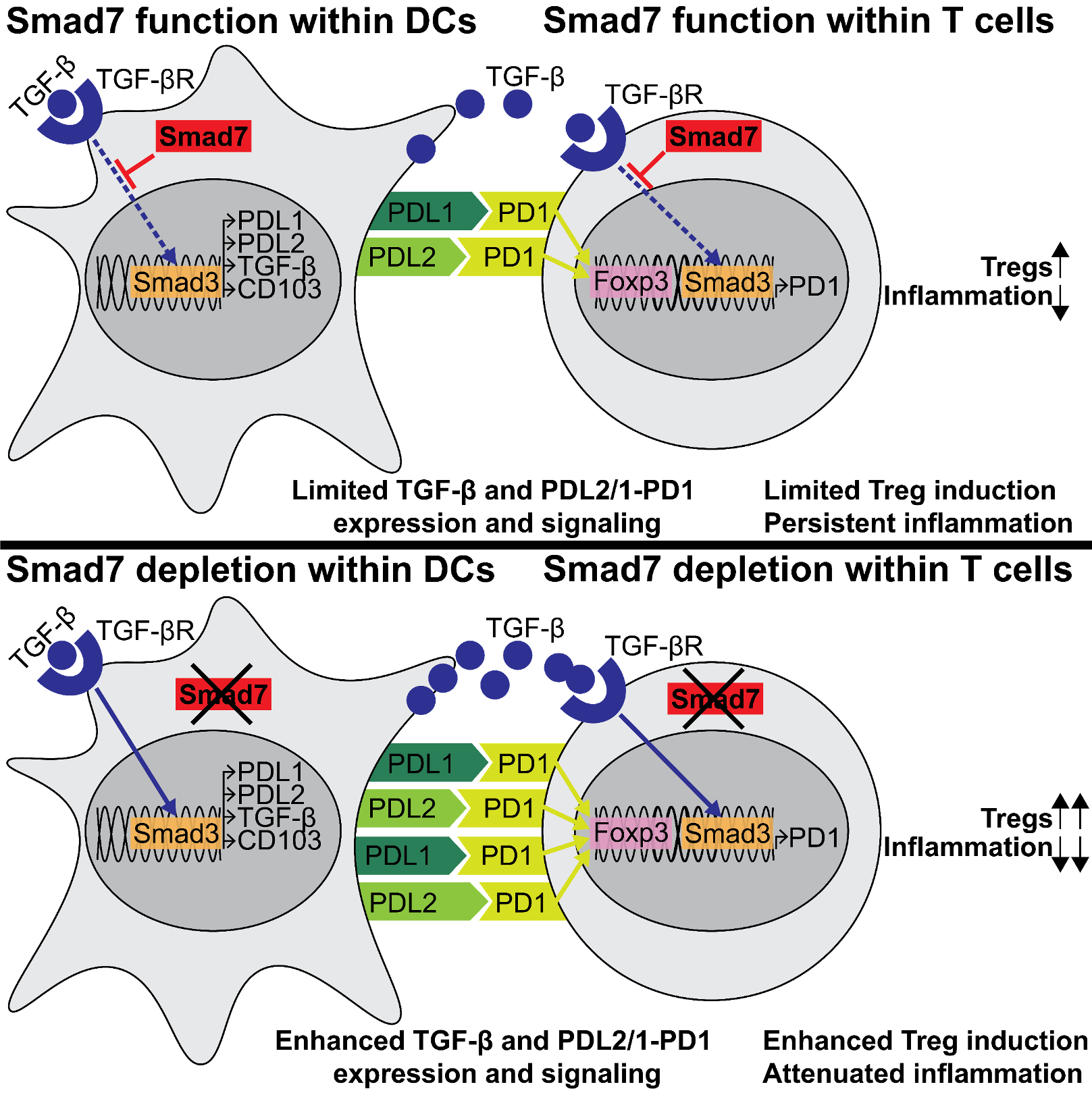
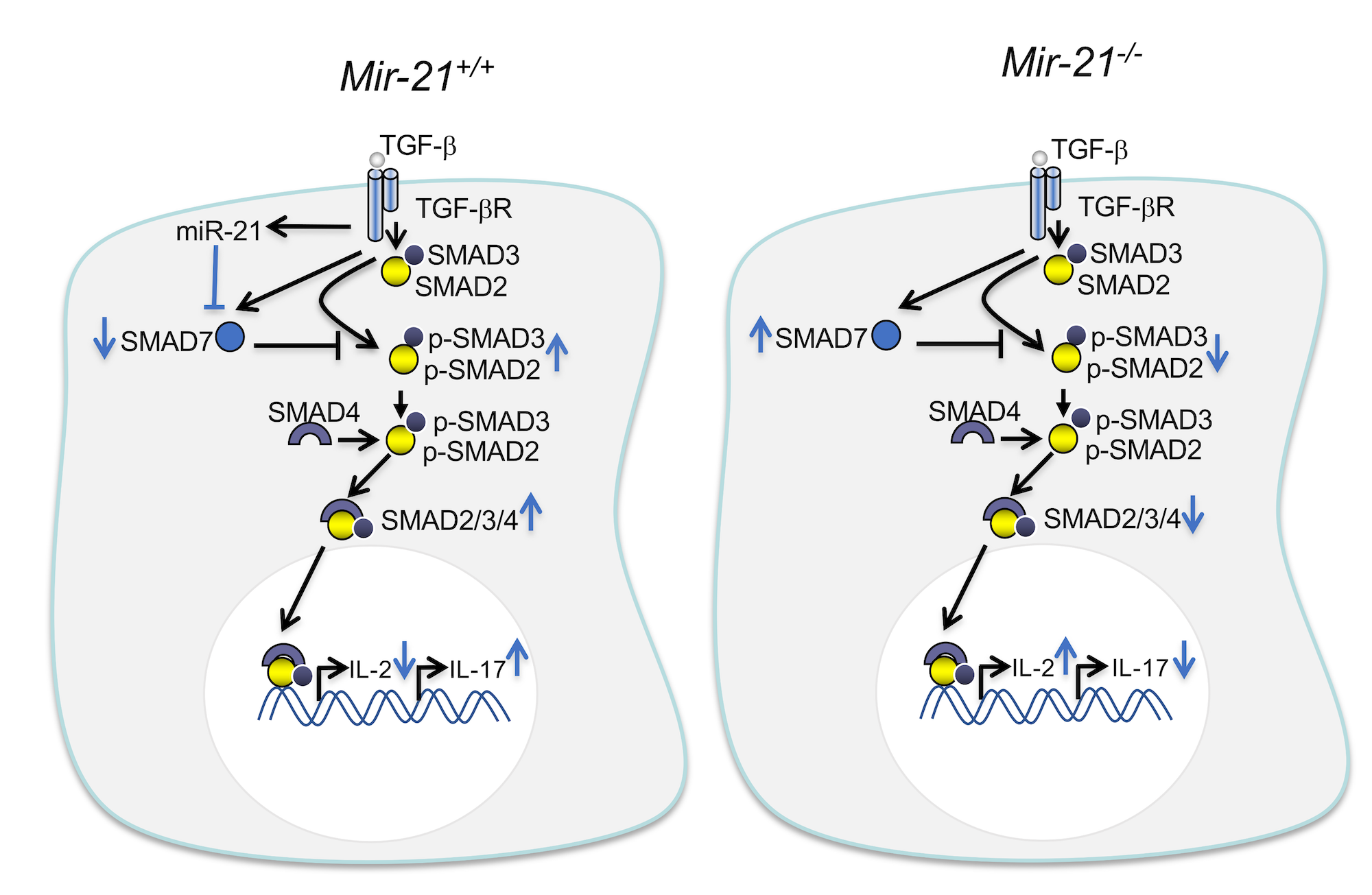
Although MS is our primary focus, we have also expanded our research to include miRNA modulation of innate and adaptive immunity in other chronic inflammatory disorders, with an interest in the gut. For this, we have established mouse models of colitis, and colitis-associated and sporadic intestinal cancer, in our lab. Recently, we characterized a novel role for Smad7, a miR-21 target which we previously identified, in promoting inflammation in inflammatory bowel disease (IBD) models. We found Smad7 exerts a pathogenic role in colitis by inhibiting immunoregulatory PDL1/2-PD1 signaling within dendritic cells (DCs) and T cells, which is known to induce Tregs. Based on these data, we tested Smad7 silencing therapy via antisense oligonucleotide in colitis and showed that it ameliorates colitis by promoting PD1-mediated induction of Tregs (Cell Reports, 2019).
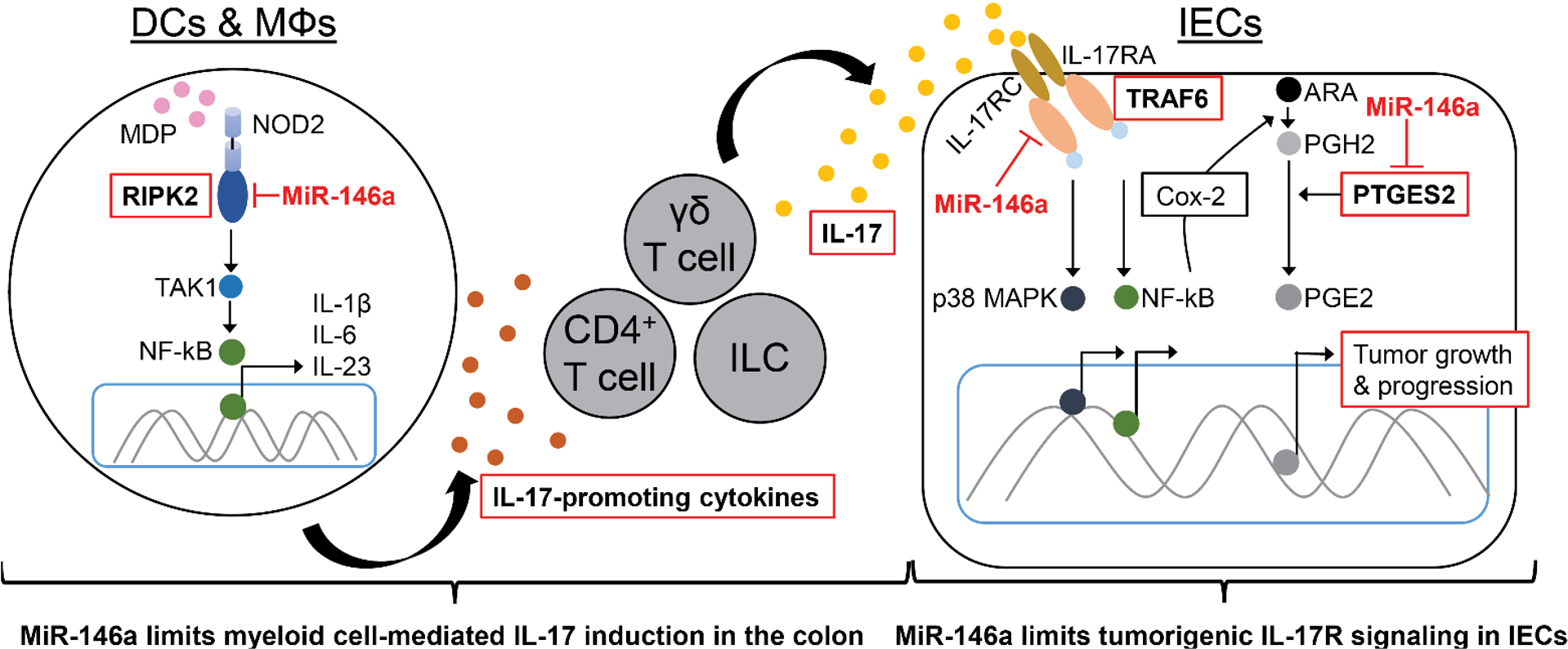
Because intestinal inflammation can lead to colorectal cancer (CRC) in animal models and IBD patients, we are also actively pursuing colorectal cancer miRNA studies. Our major aim is to identify novel mechanisms by which miRNAs regulate intestinal inflammation in CRC. Most recently, we have identified a critical regulatory role for miR-146a in preventing destructive colonic inflammation and associated tumorigenesis via modulation of IL-17 responses. Specifically, we found that miR-146a prevents intestinal inflammation and CRC by two interlinked mechanisms as follows: (1) by limiting myeloid cell-mediated inflammatory IL-17 production and (2) by inhibiting tumorigenic IL-17R signaling in IECs. Importantly, we have shown preclinical administration of miR-146a mimic or direct inhibition of miR-146a targets can ameliorate colonic inflammation and CRC (Nature Communications, 2021).